Glycoinformatics-assisted N-Glycosylation Site Analysis Service
Unleashing the Power of Precision: Your Partner in N-Glycosylation Site Analysis
N-glycosylation modification is a common post-translational way for organisms to regulate the localization, function, activity, longevity, and diversity of proteins in tissues and cells. N-glycosylation site is one of the important prerequisites for understanding the function of glycan chains, and the variability of glycosylation modification sites is a key indicator of cellular activity. Therefore a comprehensive analysis of N-glycosylation sites is of significance biologically.
At CD BioGlyco, we are proficient in Glycoinformatics methods and provide a series of Glycoinformatics-assisted Glycomics Analysis Services to our clients to help them solve the difficulties encountered in glycomics analysis. Here, the glycoinformatics-assisted N-glycosylation site analysis services we provide to our clients are shown below.

N-Glycosylation site prediction and identification
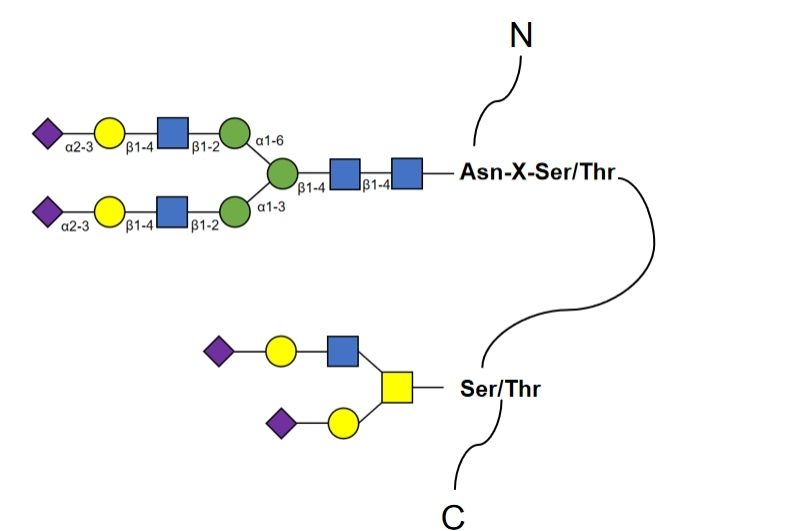
A specific glycanase (e.g., PNGase F) is used to release the glycan chain, resulting in a deglycosylation site on the peptide and a deglycosylation trace on the peptide: asparagine undergoes deamidation, and the molecular weight increases. The identification of the N-glycosylation site is obtained by liquid chromatography-mass spectrometry (LC-MS)/MS searching for asparagine undergoing deamidation and by filtration of the N-glycosylation conserved sequence Asn-X-Ser/Thr (X represents any amino acid other than proline).
N- glycosylation degree analysis
We offer radiolabeling, high-performance liquid chromatography (HPLC), or MS to quantify the degree of N-glycosylation of proteins. In addition, we offer algorithmic techniques to identify potential N-glycosylation sequence patterns to help our clients perform rapid N-glycosylation analysis.
Structural analysis
We use X-ray crystallography (XRD), nuclear magnetic resonance (NMR), ion mobility-MS (IM-MS), and microarray assay to probe where N-glycosylation sites are located in the protein structure and how glycosylation affects protein structure and stability. Among them, IM-MS allows for the analysis of isomeric glycans.
It is worth noting that we provide custom N-glycosylation site analysis solutions for both pure protein samples, such as monoclonal antibodies, and complex samples, such as whole-cell proteins.
Publication Data
Technology: MS
Journal: Scientific Reports
IF: 4.6
Published: 2018
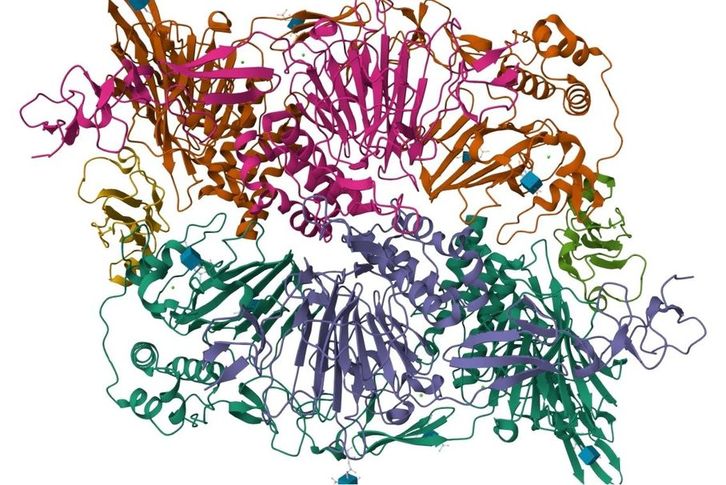
Results: In this work, the authors used MS to analyze the N-glycosylation profiles of the Fc receptor for soluble immunoglobulin G (FcγRIIIb) purified from human serum. The experimental data showed a trend of unique and common glycoforms exhibited at each N-glycosylation site between natural and recombinant FcγRIII glycoproteins. Among the N-glycosylation sites of serum-soluble FcγRIIIb glycoprotein, Asn45 was completely occupied by high mannose-type oligosaccharides, and the remaining sites were modified by complex oligosaccharides with sialic acid and fucose residues. In summary, MS-based N-glycosylation site analysis is important for optimizing the efficacy of therapeutic antibodies.
 Fig.1 MS analysis of FcγRIIIb site-specific glycoforms. (Yagi, et al. 2018)
Fig.1 MS analysis of FcγRIIIb site-specific glycoforms. (Yagi, et al. 2018)
Applications
- Glycoinformatics-assisted N-glycosylation site analysis can be used in therapeutic research of congenital disorders of glycosylation (CDGs).
- Adequate analysis of the N-glycosylation site can be used to research the effects of N-glycosylation on protein function and also enables the investigation of physiologically active processes involving N-glycosylation for cellular activity, signaling, or interaction with other proteins.
- N-Glycosylation site analysis can help to understand the nature of life phenomena, elucidate the mechanism of disease, and provide guidance for early diagnosis and treatment of diseases.
Advantages
- We provide a one-stop solution for complete analysis from N-glycan chain structure to N-glycosylation site identification and glycopeptide resolution.
- We provide various N-glycosylation site analysis methods to our clients, and we also customize the analysis solutions according to the client's specific needs.
- We provide our clients with rigorous N-glycosylation analysis reports and data interpretation, as well as direction for subsequent applications.
Frequently Asked Questions
- What is an N-glycosylation site?
- An N-glycosylation site refers to a specific amino acid residue on the surface of a protein on which an N-glycosylation modification has occurred. N-glycosylation is a common type of glycosylation modification that alters protein function, stability, localization, and interactions.
- What role does N-glycosylation play in organisms?
- N-glycosylation plays a variety of important roles in organisms, including:
-
Protein stability: N-glycosylation modifications help to maintain the stability of proteins and prevent them from being degraded.
-
Cell signaling: N-glycosylation modifications affect the structure and function of proteins, thereby modulating intercellular signaling pathways.
-
Cell adhesion and signal recognition: N-glycosylation modifications change the sugar groups on the surface of proteins, affecting their roles in cell adhesion and signal recognition.
CD BioGlyco is a specialized biology company with a long-term commitment to glycobiology research. We have developed a series of glycoinformatics technologies to provide custom N-glycosylation site analysis services to our clients all over the world. Please feel free to contact us if you are interested in our service.
Reference
- Yagi, H.; et al. Site-specific N-glycosylation analysis of soluble Fcγ receptor IIIb in human serum. Scientific Reports. 2018, 8: 2719.
For research use only. Not intended for any diagnostic use.
Quick Links
Related Services



 Fig.1 MS analysis of FcγRIIIb site-specific glycoforms. (Yagi, et al. 2018)
Fig.1 MS analysis of FcγRIIIb site-specific glycoforms. (Yagi, et al. 2018)