


Due to their participation in angiogenesis, tumor development, and metastasis, Galectin-1 (Gal-1) and Galectin-3 (Gal-3) have undergone the most extensive research. One of the strongest multivalent Gal-3 inhibitors to date was found to be a compound with a modest density of 19 conjugated asymmetric thiodigalactoside (TDGs), providing a striking example of the advantages of multivalent ligand presentation.
There have been reports of several scaffolds carrying numerous Galectin ligands so far. Bovine serum albumin (BSA) was employed as a simple protein carrier for neo-glycosylation in several earlier experiments. The most widely used amine-reactive reagents, N-hydroxy-succinimidyl (NHS)-esters, provide a wider range of amine-reactive ligands for labeling proteins. With the use of this past knowledge, researchers have now effectively developed a method for neo-glycoproteins by conjugating NHS functionalized TDG to lysine residues in BSA. The TDG-conjugates display very high inhibitory potencies despite having little to no associated ligands, which is the study's most striking conclusion. Some of the most powerful Gal-3 inhibitors were created by combining a very efficient monovalent ligand with an advantageous multivalent presentation. The multivalent TDG conjugates also serve as the first instance of TDG derivatives being used to decorate a non-glycosylated carrier.
 Fig.1 The multivalent attachment of TDG derivative using bovine serum albumin (BSA) as the scaffold. (Zhang, et al., 2018)
Fig.1 The multivalent attachment of TDG derivative using bovine serum albumin (BSA) as the scaffold. (Zhang, et al., 2018)
With low modification degrees, LacDiNAc-LacNAc conjugated BSA has a highly clear affinity for Gal-3. Gal-3 could only be addressed utilizing low-modified LacDiNAc-LacNAc conjugated BSA for anticipated applications, such as anti-cancer treatment or imaging. Squaric acid diethyl ester can be used as a linker to control the degree of LacNAc-LacNAc or LacDiNAc-LacNAc conjugation to BSA. The multivalent neo-glycoproteins can be further loaded with cytotoxic agents and tagged with fluorescent dyes to enable molecular imaging-based tumor diagnosis and tumor treatment. The custom-made neo-glycoproteins are promising targets for cancer-related biomedical research that aims to inhibit Gal-3. CD BioGlyco provides Custom Glycoprotein Synthesis including Chemoenzymatic Synthesis and Chemical Synthesis for customers to produce multivalent neo-glycoproteins.
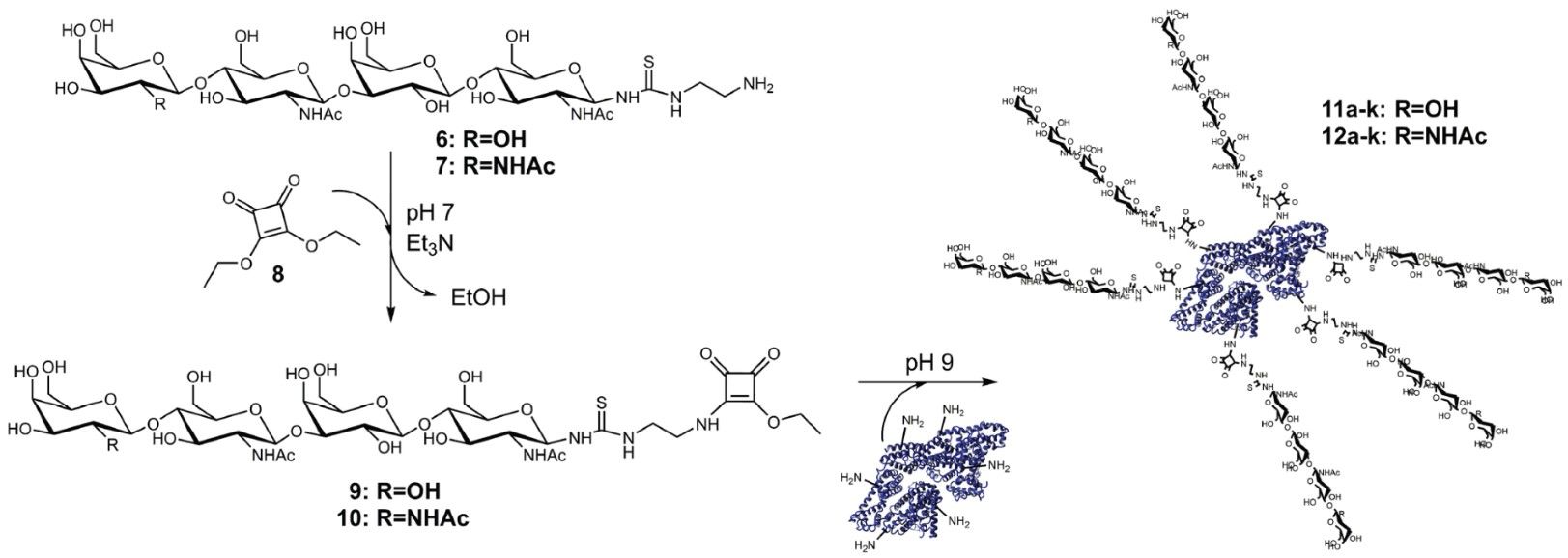 Fig.2 Two-step neo-glycoprotein synthesis. (Böcker, et al., 2015)
Fig.2 Two-step neo-glycoprotein synthesis. (Böcker, et al., 2015)
CD BioGlyco has advanced glycoprotein synthesis platforms and glycobiology microarray platforms for customers to help their research. If you are interested in our services, please contact us for more information. Moreover, we have outstanding scientists specializing in these services offered.
References
