


Label-free quantitative methods designed for glycopeptide analysis have been developed for quantifying glycosylation changes on glycoproteins. CD BioGlyco has spent quantities of funds and time in developing label-free strategies for glycomic quantitation. We look forward to becoming your scientific assistant in glycobiology.
Although glycan quantitation is a more established technology, the amount of information about glycosylation profiles that people can obtain is limited. It is well known that monitoring glycosylation profiles at a single glycosylation site are very important because the glycosylation at specific sites in proteins can regulate the structure, function, or metabolic clearance of proteins. Recently, the development of LC-MS technology has greatly improved the quantification of glycosylation site occupancy, in which labeling (including SILAC, dimethylation, TMT, and iTRAQ ) and label-free strategies based on peptide-ion intensity or spectral counting is usually applied.
The label-free strategies have the characteristics of experimental simplicity in sample preparation and have the potential to easily compare multiple samples. Comparing different sample sets through glycopeptide analysis or glycan analysis reveals that signal intensities between mass spectrum samples may be different. In order to alleviate most of the variation caused by changes in MS response among samples, data normalization has been applied to glycomics research such as dividing the ionic abundance of each glycopeptide by the total intensity of all glycopeptide peaks present in a given spectrum to produce reproducibly quantitative, label-free data.
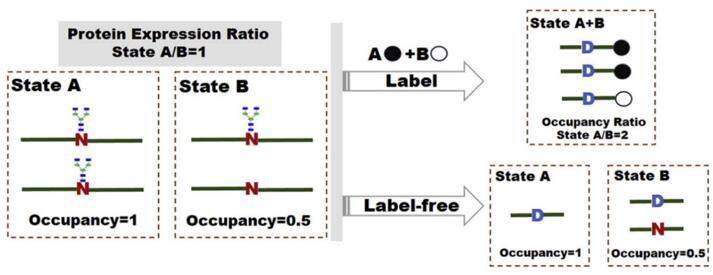 Fig.1 MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang, 2017)
Fig.1 MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang, 2017)
CD BioGlyco has developed label-free quantitative methods which have the advantages of good reproducibility, strong stability, and applicability to various types of glycoproteins. In addition, we perform several quality-control tests and normalization methods to ensure that reproducible quantitative and label-free data are produced. Services we provide include but are not limited to:
The powerful instrument capability of CD BioGlyco has the potential to expand the chemical labeling experiment and develop more label-free quantitative strategies. We are confident to meet customers' requirements for glycomic quantification. If you are interested in our services, please contact us for more information.
Reference:
