


CD BioGlyco has rich experience in the purification and structural analysis of lipopolysaccharides (LPSs), and we provide competitively advanced technology platforms. We have confidence to be your essential research assistant in the field of glycobiology.
LPSs are located on the cell surface of almost all Gram-negative bacteria, such as Escherichia coli, Klebsiella, Salmonella, Pseudomonas, etc. Its basic structure consists of three parts: lipid A, core oligosaccharides and repeating polysaccharides called O-antigens. All parts of LPS show inherent biological and chemical heterogeneity. Amphiphilic LPS molecules form aggregates in aqueous solutions, although sodium dodecyl sulfate (SDS), ethylenediaminetetraacetic acid (EDTA) or proteins (such as hemoglobin) can be used to destroy the structure of the aggregates. The diversity of molecular species still challenges the complete LPS structure analysis.
The most conserved part of LPS is lipid A. Changes in lipid A structure of lipid A, namely acylation, phosphorylation and glycosylation, will greatly affect the endotoxin activity. Lipid A may cause the mammalian immune system to lose control and trigger the biosynthesis of abnormal amounts of inflammatory mediators (such as interleukin 1), which may lead to septic shock. The carbohydrate portion of endotoxin is associated with serotype-specific immunogenicity, which is due to a single O antigen determinant or core oligosaccharide epitope. The relationship between the structure of endotoxin and its biological activity triggers the interest of more researchers to study in-depth the lipopolysaccharide structure.
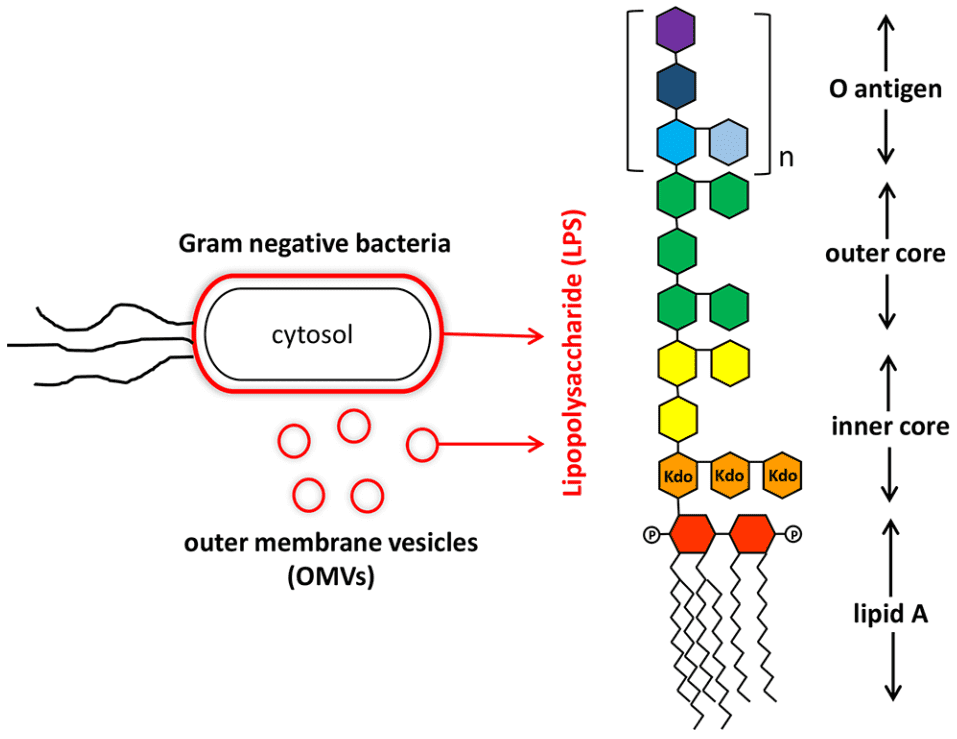 Fig 1. Structure lipopolysaccharide from Gram-negative bacteria (Lin, T.L.; et al. 2020)
Fig 1. Structure lipopolysaccharide from Gram-negative bacteria (Lin, T.L.; et al. 2020)
The analytical platform engineered for LPS scrutiny is constructed upon an amalgamation of validated and proprietary methodologies dedicated to the extraction, refinement, and structural elucidation of these intricate amphiphilic molecules. Our strategy is meticulously calibrated according to both bacterial serotype and specific experimental aims, thereby guaranteeing superior outcomes. Central to our technological repertoire are the following components:
We harness a spectrum of extraction protocols engineered to maximize both yield and purity. In the case of rough phenotypes deficient in the O-antigen domain, specialized approaches such as diethyl ether extraction are utilized for targeted molecular isolation. Our laboratory has further devised and substantiated a refined propanol-alkaline hydrolysis technique, which attains exceptional purity while mitigating hazards associated with classical protocols.
Attainment of ultrapure preparations necessitates implementing sequential purification stages. This encompasses enzymatic treatment using a combination of proteinase K, ribonuclease, and deoxyribonuclease I to eradicate residual protein and nucleic acids. Subsequent steps involve extensive dialysis and high-resolution chromatographic separations aimed at expelling lingering reagents and further refining the LPS isolate.
A battery of advanced analytical instruments is deployed to furnish exhaustive structural insights into the purified lipopolysaccharide. Modalities such as high-performance liquid chromatography (HPLC) evaluate sample homogeneity and purity, while mass spectrometric (MS) analysis delineates exact molecular mass and constitutive details—particularly within the conserved lipid A moiety. Such resolution permits the discernment of fine structural variances frequently overlooked by conventional assays.
At CD BioGlyco, we use the following methods for the structure analysis of lipopolysaccharide.
Purification and size determination by FPLC, SEC, HPAEC and RPC; monosaccharide composition by GC-MS, GC-FID and GC-CI; glycosyl linkage of neutral and acidic sugars by partially methylated alditol acetates (PMAA); elucidate glycosyl sequence and structure by NMR, MALDI-TOF MS, MS/MS and ESI-MS.
Purified by liquid-liquid extraction and HPLC; molecular weight and structure were clarified by MALDI-TOF MS, MS/MS, ESI-MS and NMR.
Clients' bacterial cells are subjected to a carefully selected extraction method tailored to the strain and LPS type (S-LPS or R-LPS). This step releases the LPS from the bacterial outer membrane.
The crude LPS extract is subjected to a multi-step purification process. This includes enzymatic digestion to remove protein and nucleic acid contaminants, followed by chemical treatments and phase separations to isolate the purified LPS fraction.
The extracted LPS undergoes final purification using advanced techniques like column chromatography. The final product is then precisely quantified and prepared for structural analysis.
The purified LPS is analyzed using our high-resolution analytical platform. This includes a battery of tests to confirm purity and homogeneity, and in-depth MS to provide a detailed structural map of the molecule, including the Lipid A, core oligosaccharide, and O-antigen components.

DOI.: 10.1051/ocl/2020025
Journal: OCL
Published: 2020
Results: The authors comprehensively analyze LPS as an essential component of gram-negative bacterial membranes. They detail the tripartite structure—lipid A (endotoxic anchor), core oligosaccharide, and O-antigen (serotype determinant)—illustrated through comparative figures. The authors emphasize LPS's dual role as both a potent endotoxin (causing septic shock at high doses) and an immune modulator (beneficial at low levels). They highlight extreme structural diversity across species, including atypical lipid A backbones (e.g., diaminoglucose in Brucella) and O-chain variability enabling bacterial adaptation. The work reviews detection methods (LAL, LC-MS, MALDI-MS) critical for pharmaceutical safety, noting advancements like Factor C alternatives. It further explores LPS's role in metabolic diseases, linking gut microbiome-derived LPS to low-grade inflammation in obesity, underscoring LPS as a key pathogenicity factor and identification tool.

The intricate architecture of LPS, particularly its polysaccharide components like the O-antigen and core oligosaccharide, underscores the critical importance of detailed structural elucidation in understanding its biological functions and interactions. This complexity highlights the need for advanced analytical techniques, which are equally vital for characterizing other clinically and industrially significant polysaccharides. Specialized Polysaccharide Analysis Services, such as Glycogen Analysis Service, Glucomannan Analysis Service, and Chitin/Chitosan Analysis Service, provide the essential expertise and technological precision required to decode the diverse structures and functions of these important biopolymers.
CD BioGlyco has first-class technology and well-trained technicians, and can provide customers with comprehensive and reliable analysis services for lipopolysaccharide research. We will continue to raise the standard to meet customers' glycobiology research needs.
Customers can contact our employees directly and we will respond promptly. If you are interested in our services, please contact us for more detailed information.
References
