


On October 2, 2025, a team from Sun Yat-sen University, led by Dong-Ming Kuang and Yuan Wei, published a study in Cell titled "Glucose restriction shapes pre-metastatic innate immune landscapes in the lung through exosomal TRAIL." This study revealed a surprising phenomenon: while a low-glucose diet or restricted Glucose Metabolism in tumor cells can inhibit primary tumor growth, it significantly promotes lung cancer metastasis through exosome-mediated immune mechanisms. This finding provides an important warning regarding current anti-cancer strategies such as starvation therapy and ketogenic diets.
Glucose metabolism is the core energy source supporting rapid tumor proliferation. Therefore, glucose deprivation (such as a low-carbohydrate diet) is considered a promising anti-tumor strategy. However, the fundamental cause of high mortality in cancer patients is not the unlimited growth of the primary tumor, but rather distant metastasis. A crucial but unresolved question is whether limiting glucose metabolism might, while inhibiting tumor growth, induce its evolution towards a more metastatic phenotype? Notably, targeting glucose metabolism not only weakens the energy supply of tumor cells but also activates various stress responses, thereby altering the composition and function of the intracellular and extracellular microenvironment. Previous studies have mostly focused on the regulation of tumor growth by glucose metabolism, neglecting its impact on the overall Microenvironment. In fact, the intense competition for glucose by tumor cells may lead to glucose deficiency in localized tumor regions, and whether these glucose-starved tumor cells affect neighboring glucose-metabolizing tumor cells through a bystander effect remains to be explored in depth.
Through large-scale clinical data analysis of 15 tumor types, the research team found that patients with low glucose metabolic activity had a significantly increased risk of recurrence within two years after surgery. Further analysis showed that liver cancer patients who developed lung metastasis within two years after surgery had significantly lower glucose metabolic activity in their tumor tissue. Using various Mouse Tumor Models, the researchers confirmed that a low-carbohydrate diet or glucose metabolism deficiency significantly promoted lung metastasis independently of primary tumor growth. Notably, this process was not due to enhanced metastatic ability of glucose-deficient tumor cells themselves, but rather through a bystander effect that promoted the spread of neighboring tumor cells with normal glucose metabolism. These findings indicate that glucose-deficient tumor cells can shape a microenvironment that significantly promotes lung metastasis of normal tumor cells.
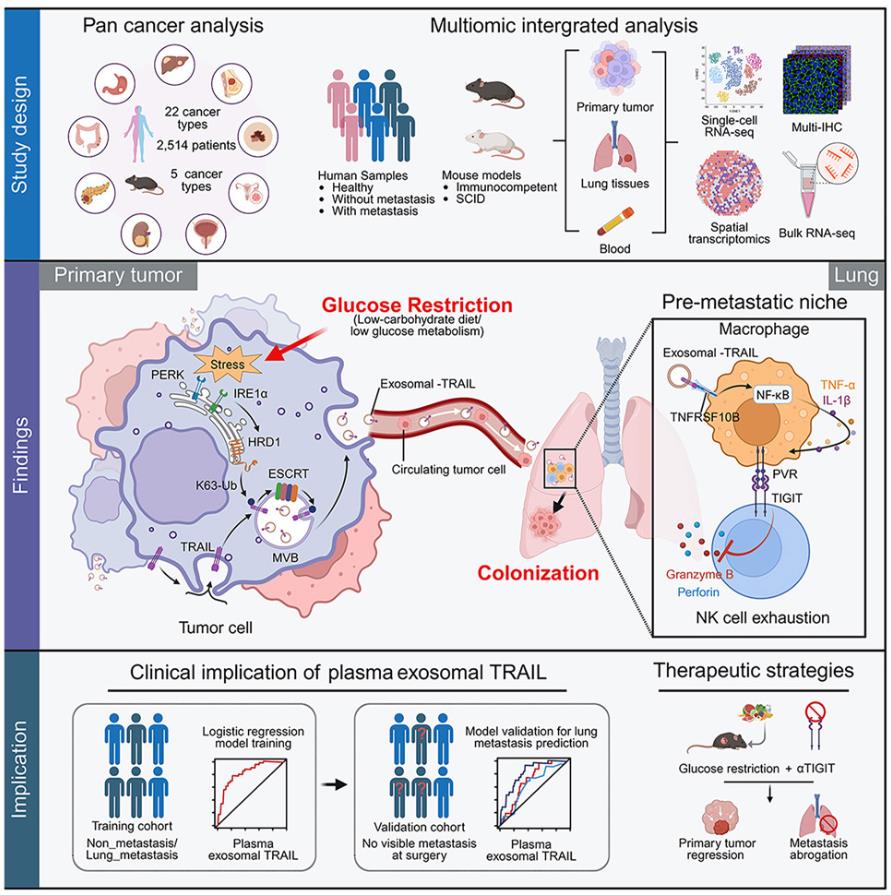
Fig. 1 Glucose restriction shapes pre-metastatic innate immune landscapes in the lung through exosomal TRAIL. (Wu, et al. 2025)
Researchers, through multi-omics analysis, animal models, and clinical samples, have for the first time elucidated the complete mechanism by which glucose restriction promotes lung cancer metastasis.
The research team further developed a method for detecting plasma exosomal TRAIL and validated its predictive value in multiple liver cancer patient cohorts. The results showed that exosomal TRAIL was significantly superior to traditional indicators such as alpha-fetoprotein and tumor size in predicting early postoperative lung metastasis. Furthermore, the study proposed that combined blockade of TIGIT signaling can effectively reverse the pro-metastatic effects induced by glucose restriction and further enhance its inhibitory effect on primary tumors, providing new therapeutic ideas for clinical practice.
This study not only reveals the complexity and potential risks of glucose metabolism intervention in Cancer Treatment but also provides a new perspective for understanding the interaction between nutrition, metabolism, and the immune system in tumor progression. In the future, early warning based on exosomal TRAIL and combination therapy targeting the PVR-TIGIT axis are expected to become new strategies for preventing tumor metastasis, promoting the precise development of tumor metabolic immunotherapy.
Reference








