


Imagine a city where the waste disposal system suddenly stops working overnight—streets are piled high with garbage, sewage overflows, and the entire system grinds to a halt. This is a true reflection of what happens to neurons in our brains under inflammatory stress.
The brain's "garbage collectors"—proteasomes—are supposed to maintain cleanliness and order within cells. However, in neurodegenerative diseases such as multiple sclerosis (MS), they undergo a replacement, where the original normal proteasome core (such as PSMB5) is replaced by an immunoproteasome core (PSMB8) due to inflammatory signals. This seemingly adaptive response of the immune system triggers a metabolic catastrophe and a storm of neuronal death.
This groundbreaking research, published in Cell in June 2025 by Professor Manuel A. Friese's team, titled "The immunoproteasome disturbs Neuronal Metabolism and drives neurodegeneration in multiple sclerosis," reveals a surprising mechanism: how the immunoproteasome PSMB8 disrupts metabolism in neurons, leading to oxidative damage, ferroptosis, and ultimately driving neurodegeneration in multiple sclerosis.
In a healthy state, neurons rely on a proteasome core subunit called PSMB5, which maintains metabolic homeostasis and antioxidant balance. However, during an MS attack, inflammatory signals, particularly IFNγ (interferon-γ), rapidly take over, inducing neurons to express the immunoproteasome PSMB8.
The study found that this replacement, seemingly a measure to enhance immunity, actually has the opposite effect in neurons—it weakens the activity of the entire proteasome, causing certain metabolic factors that should be cleared, such as PFKFB3, to accumulate, disrupting metabolic balance.
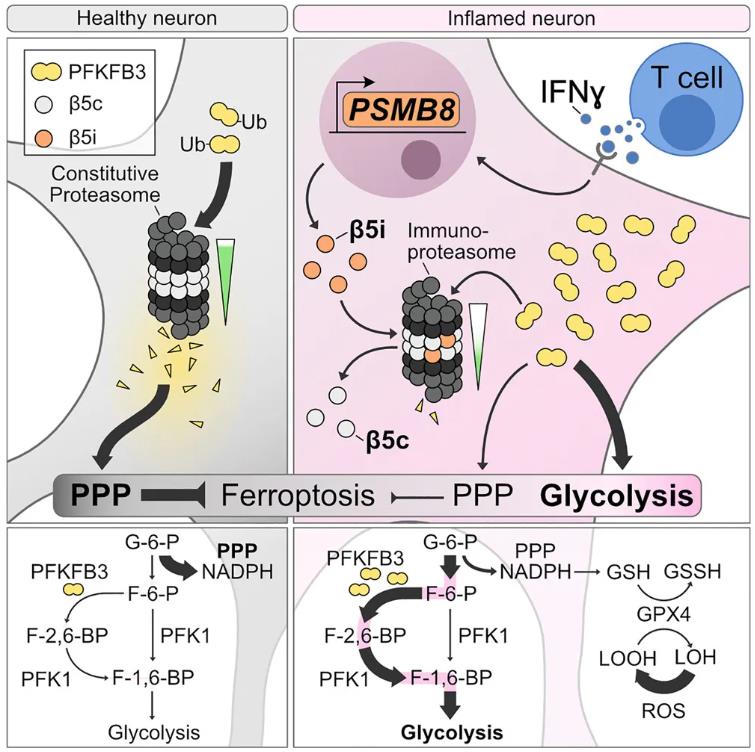
Fig. 1 The immunoproteasome disturbs neuronal metabolism and drives neurodegeneration in multiple sclerosis. (Woo, et al. 2025).
PFKFB3 may be an unfamiliar name, but it plays the role of an "energy-saving switch" in cellular energy metabolism. In neurons, PFKFB3 is usually rapidly degraded because neurons prefer another antioxidant pathway—the pentose phosphate pathway (PPP). However, when PSMB8 takes over, PFKFB3 accumulates uncontrollably. The research team discovered that this accumulation directly leads to a metabolic pattern in neurons characterized by high glycolysis and low antioxidant activity. This not only reduces the production of NADPH and glutathione (GSH) but also leaves neurons vulnerable to oxidative stress, ultimately making them more susceptible to ferroptosis, a novel cell death pathway.
Notably, the expression of PSMB8 is not merely a trigger for metabolic dysregulation; it also causes a complete collapse of Protein Degradation.
By measuring the accumulation of ubiquitin polymers (K48-Ub), researchers found that when PSMB8 is active, a large amount of undegraded waste proteins accumulate in neurons. This metabolic congestion is closely related to synaptic loss, mitochondrial structural disintegration, and signal transmission disorders in neurons.
Even more surprisingly, all of this is unrelated to the classic CDK5/CDH1 metabolic regulatory axis—meaning this is not a bypass accident of the traditional pathway, but a completely new pathogenic logic.
The research team not only found conclusive evidence of elevated PSMB8 and PFKFB3 expression in experimental EAE mouse models but also in the brain tissue of human MS patients, particularly in chronically active lesions and seemingly normal gray matter regions. This suggests that even if imaging does not show lesions, the metabolic crisis has already silently spread.
Importantly, neuron-specific knockout of PSMB8 or PFKFB3 significantly reduced neurodegeneration, synaptic damage, and oxidative stress in mice, providing new targets for precise intervention.
In neurons with upregulated PSMB8 and PFKFB3, the antioxidant defense line is gradually weakened, GPX4 activity is limited, and GSH is deficient, making neurons more susceptible to lipid peroxidation under oxidative stress, leading to Ferroptosis.
The authors successfully reversed the metabolic imbalance and cell death caused by PSMB8 and PFKFB3 by applying antioxidants (such as Q10, ferrostatin-1) and metabolic inhibitors (such as Pfk-158, 2-DG), demonstrating clear intervention potential.
This study breaks through the traditional linear framework of "inflammation-immunity-neuronal damage," using the remodeling of proteasome subtypes as a starting point to construct a closed-loop pathological mechanism encompassing Immune Regulation, protein homeostasis disruption, and metabolic reprogramming. Starting with IFNγ-mediated PSMB8 upregulation, the authors revealed that its replacement of the conventional subunit PSMB5 in neurons actually leads to a decrease in overall proteasome activity, subsequently causing the abnormal accumulation of the key glycolytic regulatory factor PFKFB3.
This change triggers enhanced glycolysis and inhibition of the pentose phosphate pathway (PPP), weakening the production of NADPH and glutathione (GSH) in the antioxidant system, ultimately leading to a significant increase in neuronal sensitivity to oxidative stress and ferroptosis, and exhibiting structural damage and functional loss. More importantly, this mechanism is not only validated in a multiple sclerosis model but may also represent the molecular basis of common vulnerability in neurons in other neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis, providing important insights for the future development of broad-spectrum neuroprotective strategies targeting proteasome and metabolic regulation.
This paper not only provides two key targets (PSMB8 and PFKFB3) but, more importantly, points out feasible intervention windows. Both known small molecule inhibitors (such as ONX-0914 and Pfk-158) and their protective effects in in vivo and in vitro experiments are clearly identified.
At the clinical level, how can Proteasome Subtype Regulation be incorporated into precision treatment strategies for neurodegenerative diseases such as MS? Is it possible to design neuron-specific proteasome inhibitors to avoid side effects on the immune system? These are all questions worth exploring in depth.
Reference








