


In nature, the ability to catalyze reactions has long been considered the exclusive domain of proteins and ribozymes. However, a recent breakthrough study demonstrates that Rationally Designed Carbohydrate Molecules can also efficiently catalyze chemical reactions. This achievement was published on February 26, 2025, in Nature by Martina Delbianco and Kaimeng Liu from the Max Planck Institute of Colloids and Interfaces. The article reports for the first time a glycan foldamer capable of catalyzing the Pictet–Spengler reaction, not only expanding the design boundaries of functional oligomers but also providing a new perspective on the potential catalytic roles of carbohydrates in biological systems.
The research team's design inspiration came from the widely existing Sialyl Lewis X Antigen in nature. This glycan structure has a rigid turn motif composed of galactose (Gal), N-acetylglucosamine (GlcNAc), and fucose (Fuc), and its folded conformation is stabilized by an unconventional hydrogen bond.
The researchers made key modifications based on the natural structure:
The resulting tetrasaccharide scaffold (4mer-I) can both fold into a stable conformation and possess substrate recognition capabilities.
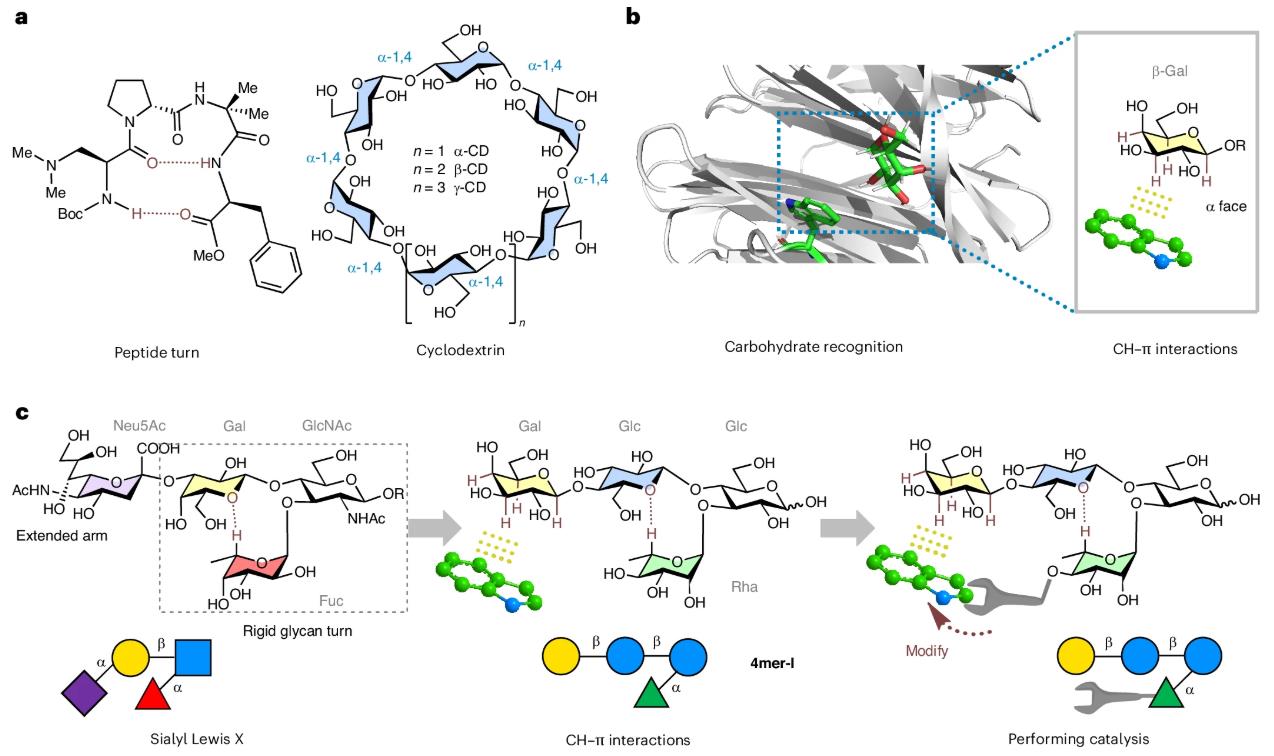
Fig. 1 Design of a functional glycan foldamer. (Liu, et al. 2025)
The CH–π interaction between sugars and aromatic rings is a core binding mechanism in many sugar-binding proteins. This study innovatively reverses this principle, using the CH bonds of the glycan chain as grippers to actively recruit aromatic substrates and position them near the catalytic center. Nuclear magnetic resonance (NMR) experiments showed that 4mer-I adopts a rigid Folded Conformation in solution, with its galactose unit's α-face oriented inwards. When co-incubated with tryptophan (Trp), significant chemical shift changes were observed in the protons of the galactose unit, confirming a specific CH–π interaction. This interaction was significantly weakened when another glucose unit was introduced on the α-face of the Galactose for steric shielding (resulting in 5mer), further validating the specificity of the recognition.
After ensuring substrate recognition capabilities, the research team introduced three different acidic catalytic groups at the C-4 position of rhamnose:
Synthesis was carried out using efficient automated glycan assembly (AGA) technology, with post-modification performed directly on the solid support, ultimately yielding the target glycan foldamers with a total yield of 28–40%. NMR analysis confirmed that these modifications did not disrupt the overall folded conformation of the glycan chain, and some acidic groups even enhanced the folding rigidity through electronic effects.
The Pictet–Spengler reaction was chosen as a model reaction—an important method for constructing alkaloid skeletons, but typically slow in aqueous solutions. In the reaction of tryptophan with propionaldehyde:
Key control experiments demonstrated:
This indicates that the CH–π interaction is crucial for accelerating the reaction—it fixes tryptophan near the catalytic carboxylic acid, significantly increasing the reaction probability.
Kinetic analysis further showed that the catalytic pathway of 4mer-IV was significantly faster than that of control groups using small molecule acids such as acetic acid or glycolic acid, highlighting its advantages as a structured catalyst.
The study also successfully applied this catalytic system to the N-terminal modification of tryptophan-containing Peptide Chains, which was effective in both water and buffer solutions, and the rate was superior to that of small molecule acid catalysis. This provides a new tool for selective protein functionalization, especially for the specific labeling of tryptophan.
This research demonstrates for the first time that:
This not only provides a new type of aqueous catalyst for chemical biology but also raises a profound and forward-looking question: could glycan chains also play undiscovered catalytic roles in natural biological systems? The abundant charged groups on natural glycan chains may regulate their reactivity through interactions with substrates, opening up a completely new research direction for glycoscience.
With the continuous progress of Glycan chemical Synthesis technology and in-depth mechanistic research, we can expect to see more glycan-based catalysts with diverse functions in the future, potentially giving rise to a completely new field of glycocatalysis.
Reference








